Product Description
INTENDED USE
The COVID-19 Neutralizing Antibody Rapid Test is a lateral flow chromatographic immunoassay for qualitative detection of neutralizing antibody to SARS-CoV-2 in human whole blood,serum,or plasma as an aid in the evaluation of human anti-SARS-CoV-2 neutralizing antibody titer.
SUMMARY
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped non-segmented positive-sense RNA virus. It is the cause of COVID-19, which is contagious in humans. SARS-CoV-2 has several structural proteins including spike(S),envelope (E), membrane (M) and nucleocapsid (N). The spike protein(S)
contains a receptor binding domain (RBD), which is responsible for recognizing the cell surface receptor, angiotensin converting enzyme-2 (ACE2). It is found that the RBD of the SARS-CoV-2 S protein strongly interacts with the human ACE2 receptor leading to endocytosis into the host cells of the deep lung and viral replication. Infection with the SARS-CoV-2 or SARS-CoV-2 Vaccine immunization initiates an immune response to produce antibodies which provide protection against
future infections from viruses. Neutralizing antibodies block the SARSCoV- 2 spike protein bind to host ACE2 receptor-binding domain show promise therapeutically and efficiency protectively.
PRINCIPLE
COVID-19 neutralizing antibody Test is a chromatographic
immunoassay for the detection of neutralizing antibodies to COVID-19
in human whole blood, serum and plasma. The test device contains:1)
Colloidal gold-labeled recombinant SARS-CoV-2 RBD antigen; 2)
Detection line (T line) immobilized with SARS-CoV-2 RBD antigen for
detecting COVID-19 neutralizing antibody and Control line(C line)
immobilized with quality control antibody on nitrocellulose membrane.
If the sample contains COVID-19 neutralizing antibody, it will bind to
antigens coated on colloidal gold, and a pink colored band will develop
in the test region (T) on the membrane.If there is no COVID-19
neutralizing antibody in the sample, the pink colored band in the test
region (T) will not appears and that suggests a negative result.
The control line(C line)should be appears regardless of whether the
test line (T line)appears.If the control line(C line) does not appear, the
test result is invalid, and the sample needs to be tested again with
another test kit.
SPECIMEN COLLECTION AND PREPARATION
1.The COVID-19 neutralizing antibody Rapid Test is intended for use with human whole blood, serum or plasma specimens only.
2.Only clear, non-hemolyzed specimens are recommended for use with this test. Serum or plasma should be separated as soon as possible to avoid hemolysis.
3.Perform testing immediately after specimen collection. Do not leave specimens at room temperature for prolonged periods. Serum and plasma specimensmaybestoredat2-8°C for up to 3 days. For long term storage, serum or plasma specimens should be kept below -20°C. Whole blood collected by venipuncture should be stored at 2-8°C if the test is to be run within 2 days after collection. Do not freeze whole blood specimens.Whole blood collected by finger stick should be
tested immediately.
4.Containers containing anticoagulants such as EDTA, citrate, or heparin should be used for whole blood storage.
5.Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Avoid repeated freezing and thawing of specimens.
6.If specimens are to be shipped, pack them in compliance with all applicable regulations for transportation of etiological agents.
7.Icteric,lipemic,hemolyzed,heat treated and contaminated sera may cause erroneous results.
8.When collecting finger stick blood with a lancet and alcohol pad, Please discard the first drop of whole blood.
MATERIALS
Materials provided
• Test Devices •Buffer
• Disposable plastic pipette • Package insert
• Lancets (for finger stick whole blood only) (optional)
• Alcohol pad (optional)
Materials required but not provided
• Specimen collection containers
• Centrifuge (for plasma only)
• Micro pipette
• Timer
DIRECTIONS FOR USE
Allow the test device,specimen,buffer,and/or controls to reach room temperature (15-30°C) prior to testing.
1. Bring the pouch to room temperature before opening.Remove the test device from the sealed pouch and use it as soon as possible.
2. Place the test device on a clean and level surface.
3. Add 20μl whole blood or 10μl serum and plasma into the sample well of the test device, then add 80μl buffer into the
sample well.
4. Read the result at 15~20 minutes. The result is invalid after 20 minutes.
Note: 1 drop volume of the disposable plastic pipette provided is about 10μl.
INTERPRETATION OF RESULTS
NEGATIVE:Presence of a single colored band in the control region (C) indicates the absence of COVID-19 neutralizing antibodies or that the concentration of antibodies in sample is below the detection cut-off level.
POSITIVE: Presence of two visible, pink-colored bands, one in the control region (C) and one in test region (T), indicates
presence of COVID-19neutralizing antibodies in sample.
INVALID: There is no line appeared in the C region.
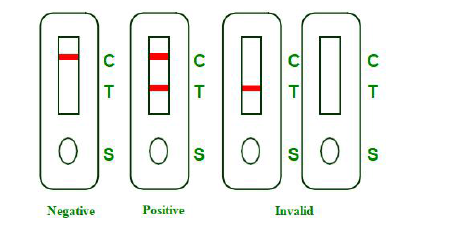
Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure.Review and repeat the procedure with a new test device. If the problem
persists,discontinue using the test kit immediately and contact your local distributor.
QUALITY CONTROL
Internal procedural controls are included in the test. A color line appearing in the control region(C) is an internal positive
procedural control.It confirms sufficient specimen volume and correct procedural technique. Control standards are not supplied with this kit;however,it is recommended that positive and negative controls be tested as a good laboratory practice to confirm the test procedure and to verify proper test performance.
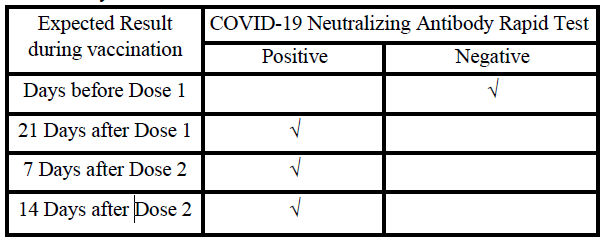
EXPECTED RESULTS AFTER VACCINATION
The SARS-CoV-2 neutralizing antibody is produced by the human body after natural immunity and vaccine injection, and protects the
human body from SARS-CoV-2 infection. Based on the SARS-CoV-2
vaccine procedure, the recommendations for using this reagent to
monitor the content of SARS-CoV-2 neutralizing antibodies in the
human body are as follows:
PERFORMANCE CHARACTERISTICS Sensitivity and Specificity
The COVID-19 Neutralizing Antibody Rapid Test has been compared to the leading commercial COVID-19 IgG Rapid Test Device using clinical specimens from naturally infected patients.
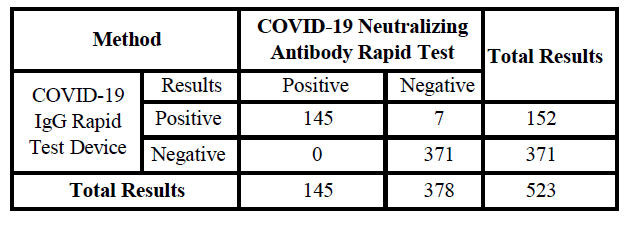
Relative Sensitivity:145/152=95.39%
Relative Specificity:371/371=>99.9%
Cross-reactivity
The COVID-19 Neutralizing Antibody Rapid Test Device has been tested foranti-influenza A virus, anti-influenza B virus, anti-RSV, anti-Adenovirus, HBsAg, anti-Syphilis, anti-HIV, anti-rheumatoid factor, anti-M.pneumonia, anti-chlamydiapneumonia and anti-HCV positive specimens. The results showed no cross-reactivity.
Interfering Substances
The following compounds have been tested using the COVID-19 Neutralizing Antibody Rapid Test Device and no interference was observed.
Triglyceride:5000mg/dL Ascorbic Acid:
20mg/dL Hemoglobin: 1000mg/dLBilirubin: 60mg/dL
Oxalic acid: 100mg/dL Human serum albumin 2000mg/dL
PRECAUTIONS
1.For professional In Vitro Diagnostic Use Only.Do not use the kit beyond the expiration date.
2.Do not eat, drink or smoke in the area where the specimens or kits are handled.
3.Do not use the test if the pouch is damaged.
4.Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout testing and follow the standard procedures for proper disposal of specimens.
5.Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are being tested.
6.The used test should be discarded according to local regulations.
7.If the concentration of neutralizing antibodies falls below the detection limit, the result will show negative.
8.If you have received the vaccine but the result show negative, please combined other relevant tests.
LIMITATION OF USE
1.The accuracy of the test depends on the sample collection process.
Improper sample collection, improper storage of samples, stale samples, or repeated freeze-thaw cycles of samples will affect the test
results.
2.The test result of this kit is for clinical reference only and should not be used as the sole basis for clinical diagnosis and treatment. The clinical management of patients should be considered in combination with their symptoms/signs, medical history, other laboratory tests, and treatment responses.
3.It is recommended to review the suspicious negative results by using combined other relevant tests.
SYMBOLS
If the concentration of neutralizing antibodies in the sample falls below the detection limit, a negative result will occur.
 Euro
Euro
 British Pound
British Pound
 US Dollar
US Dollar








